
Robert F. Borkenstein’s breathalyzer was a remarkable invention in the field of road safety. It used a simple color-changing chemical reaction to save many lives and keep the roads safer.
Drinking and then getting on the road home wasn’t a major issue during the 1800s. How badly could drunk bicyclists or pedestrians hurt themselves or others while making their way home?
However, Carl Benz introduced the world to the first commercially available automobile in the 1880s, and within a decade, the situation of drinking and driving had turned into a problem.
Then, in the summer of 1954, from the depths of Robert F. Borkenstein’s basement, rose our hero—the Breathalyzer™.
Breath alcohol analyzers are brilliant pieces of equipment that use simple chemistry to save thousands of lives every year. So, let’s get give them their due by getting a bit more acquainted with their origin story and functionality.
But first, we should understand why alcohol makes people menacing drivers in the first place.

Recommended Video for you:

Driving requires us to multitask, be alert, remain aware of our surroundings, process information, and act with minimal reaction time. To sum up, a driver needs to have complete control over their senses, but alcohol undermines those senses. Alcohol, instead of getting digested like food, diffuses into the bloodstream through the walls of the stomach and intestines. Once in our body, it goes wherever blood does and gets absorbed anywhere that water is present (since alcohol is highly water-soluble). Thus, all the major organs, including the heart, brain, lungs and muscles, will reach the same concentration of alcohol as that of our blood. After entering the brain, alcohol enhances the effects of an important inhibitory neurotransmitter, GABA. As a result, our brain cells show delayed response and communication. Alcohol also dampens the efficiency of the cerebellum, thereby impairing clear vision and motor skills. Read more.
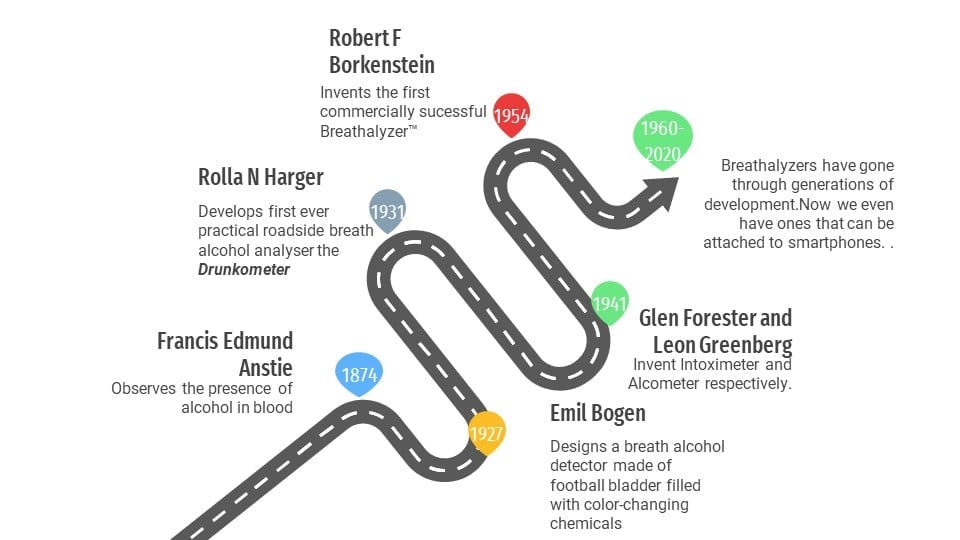
In the early 1900s, the only way to detect drunk drivers was to look for the tell-tale signs of drunken driving, such as swerving, excessive speeding, bloodshot eyes, etc. One could also conduct field sobriety tests, which are still in practice today. However, this system was completely dependent on a cop’s intuition or perception. There were no scientific standards to determine the level of inebriation. Scientists and authorities eventually came together to find a way to standardize when a person could be charged for “driving under the influence” or DUI. There are many compact and accurate breath alcohol testers available on the market today, but we are going to look at the one that went on to become synonymous with breath alcohol analyzers, the Breathalyzer™. The inventor of the breathalyzer, Robert F. Borkenstein, was a captain at the Indiana state police’s laboratory services, and he later became a professor at Indiana University. He collaborated with Hagner during his work on the Drunkometer and was trained on how to operate it. This training piqued his interest in breath alcohol analyzers. Since he worked with the police force, he wanted to build something more precise and easier to operate, something that could prove to be a more convenient successor to Hagner’s Drunkometer. After years of trial and error, in the year 1954, he came up with a form for the revolutionary breathalyzer™—the technology that started it all. Also Read: Why Is Alcohol So Obvious On A Person’s Breath? How To Get Rid Of It?
The subject (person suspected to be drunk) is asked to blow through a tube into the contraption. They are asked to provide a deep exhale, in order to obtain alveolar or deep lung breath. The operator checks for a deflection in the indicator needle. If the needle moves, it is then brought back to zero with the help of a knob. The demarcation on the knob helps the police ascertain if a person is drunk or not.  The air from the driver’s lungs enters the test vial, which contains a reddish-orange mixture consisting of potassium dichromate, sulphuric acid, and silver nitrate (catalyst). This mixture, along with the ethanol (the type of alcohol found in alcoholic beverages) from one’s breath, forms a redox reaction system. In a redox reaction, one of the reactants gets oxidized simultaneously reduces another. The one that is getting oxidized either gains oxygen or attains a higher oxidation state, while the species getting reduced loses oxygen or acquires a lower oxidation state. The sulphuric acid (H2SO4) in the solution helps in the absorption of ethanol (chemical formula: C2H5OH) from the air into the mixture. Potassium dichromate (K2Cr2O7), loses an oxygen atom upon reacting with the -OH group present in ethanol. Chromium in potassium dichromate has an oxidation of +7, but after getting reduced by ethanol, is at a +3 oxidation state. It further reacts with the sulfate ions (SO4 2 -) from sulphuric acid to form chromium sulfate and potassium sulfate.
The air from the driver’s lungs enters the test vial, which contains a reddish-orange mixture consisting of potassium dichromate, sulphuric acid, and silver nitrate (catalyst). This mixture, along with the ethanol (the type of alcohol found in alcoholic beverages) from one’s breath, forms a redox reaction system. In a redox reaction, one of the reactants gets oxidized simultaneously reduces another. The one that is getting oxidized either gains oxygen or attains a higher oxidation state, while the species getting reduced loses oxygen or acquires a lower oxidation state. The sulphuric acid (H2SO4) in the solution helps in the absorption of ethanol (chemical formula: C2H5OH) from the air into the mixture. Potassium dichromate (K2Cr2O7), loses an oxygen atom upon reacting with the -OH group present in ethanol. Chromium in potassium dichromate has an oxidation of +7, but after getting reduced by ethanol, is at a +3 oxidation state. It further reacts with the sulfate ions (SO4 2 -) from sulphuric acid to form chromium sulfate and potassium sulfate. 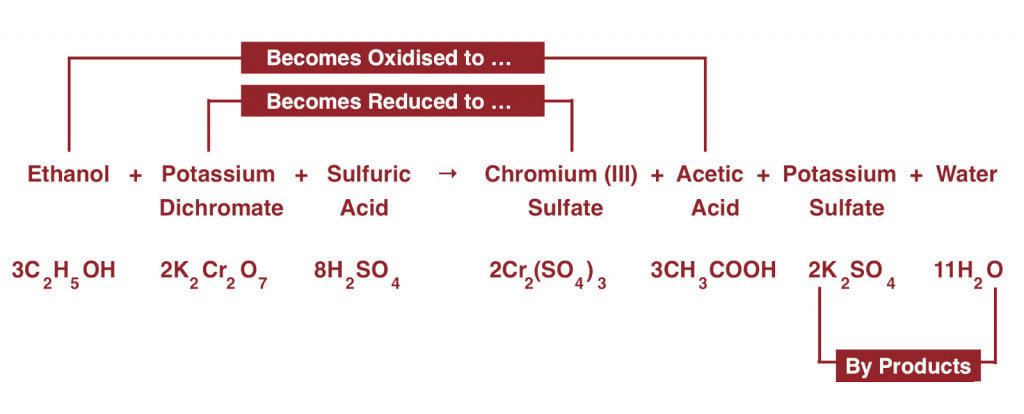 Ethanol, after gaining an oxygen atom from potassium dichromate, gets oxidized into acetic acid (CH3COOH) and water (H2O). The most fascinating thing about this reaction is that it’s a color-changing reaction. The potassium dichromate solution, which is primarily reddish-orange, in the presence of sulphuric acid and ethanol, gives rise to a green-colored chromium sulfate. Inside the breathalyzer is a standard vial that has a mixture identical to the test vial, but without any ethanol. There is a light source that illuminates both the vials, and at the other end there is a photocell system that senses the transmittance from both the vials. The difference in transmittance is observed and the quantitative analysis is done according to Beer-Lamberts Law (which states a correlation between the properties of a colored solution and the weakening of light passing through it). The difference in transmittance of the test and standard vials is dependent on the change in color from reddish-orange to green. The formation of green chromium sulfate depends on the amount of ethanol present in the reaction.
Ethanol, after gaining an oxygen atom from potassium dichromate, gets oxidized into acetic acid (CH3COOH) and water (H2O). The most fascinating thing about this reaction is that it’s a color-changing reaction. The potassium dichromate solution, which is primarily reddish-orange, in the presence of sulphuric acid and ethanol, gives rise to a green-colored chromium sulfate. Inside the breathalyzer is a standard vial that has a mixture identical to the test vial, but without any ethanol. There is a light source that illuminates both the vials, and at the other end there is a photocell system that senses the transmittance from both the vials. The difference in transmittance is observed and the quantitative analysis is done according to Beer-Lamberts Law (which states a correlation between the properties of a colored solution and the weakening of light passing through it). The difference in transmittance of the test and standard vials is dependent on the change in color from reddish-orange to green. The formation of green chromium sulfate depends on the amount of ethanol present in the reaction.  The equipment compares the difference in transmittance and uses the Beer-Lamberts Law to determine the concentration of the green substance, which indirectly indicates the concentration of alcohol in the breath.
The equipment compares the difference in transmittance and uses the Beer-Lamberts Law to determine the concentration of the green substance, which indirectly indicates the concentration of alcohol in the breath.
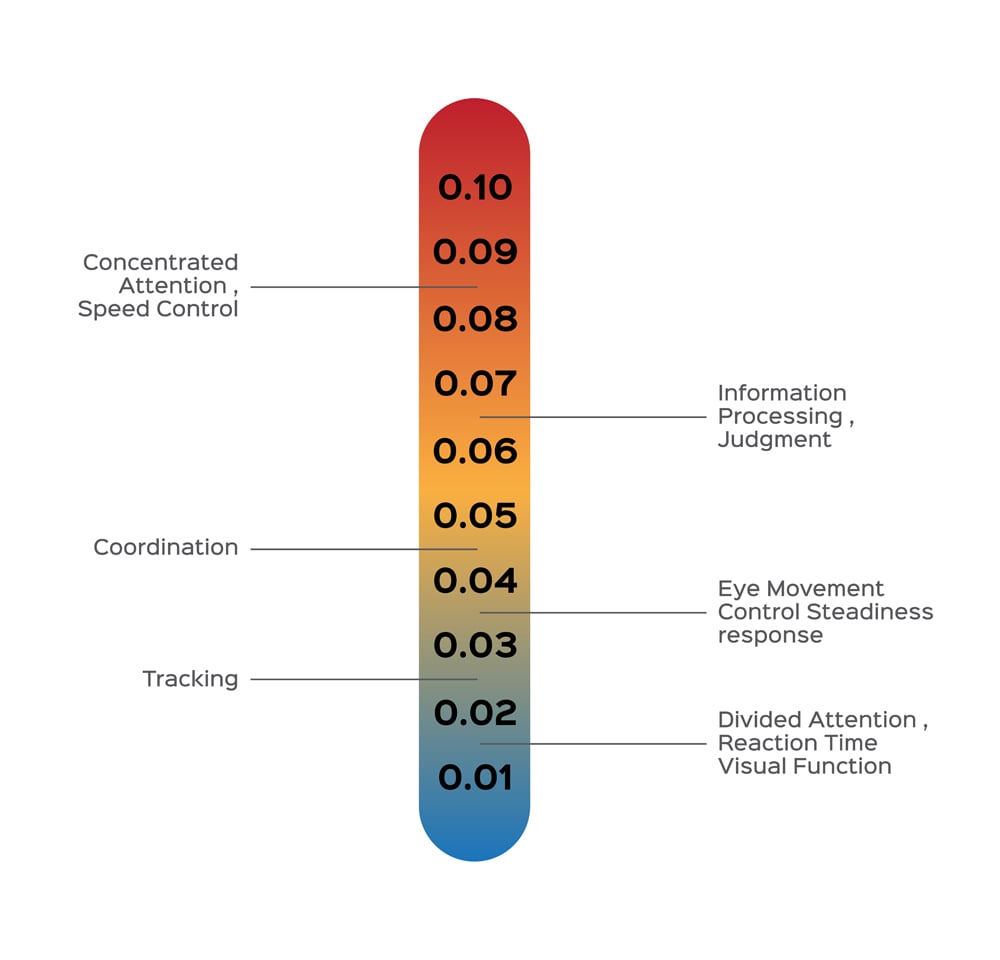
Yes, alcohol in a person’s breath does indicate the presence of alcohol in their blood, but the concentrations are different. When a person consumes alcohol, it enters the bloodstream and moves through the entire body. During the trip, it gets distributed in the lung tissues and with the air in one’s lungs. Breath analyzers use an indirect method of calculating blood alcohol concentration (BAC) based on Henry’s Law. Henry’s Law states that for a closed system at constant temperature and pressure, the concentration of a volatile substance dissolved in a solution is proportional to the concentration of the same substance in the air above the solution. A human body meticulously regulates its temperature and pressure. Now, if we consider the body as a closed system, then the concentration of alcohol in the air present in a person’s lungs is in proportion to the concentration of alcohol in their blood. Research has shown that the ratio is 2100:1 (i.e., for every 1 ml of alcohol in breath, there is 2100 ml of alcohol in the blood). This ratio is not universal for every human body, but it’s considered an agreeable ratio. Drunkenness caused by alcohol varies from one gender to another, and it is also dependent on body weight. All these factors are taken into account while calculating the BAC before charging a person with a DUI. The highest BAC allowed in India is 0.03% (0.03 gms of alcohol per 100 ml of blood), 0.08% in the USA, 0.05% in western Europe. Many countries, such as Hungary, Japan and Iran have a zero-tolerance policy when it comes to drunken driving. Drunk driving policies and penalties vary from across the globe. To learn more, click here. Also Read: Why Does Alcohol Affect Women More Than Men?
About the Author
Sanjukta Mondal is a Chemist (Master of Science) from Ramaiah University of Applied Sciences (India). She likes to experiment not only in the laboratory but also in the kitchen and behind the lens. She loves watching sci-fi and live-action superhero shows and continues to watch them painstakingly even after the quality degrades. She likes to explore tales associated with complex phenomena to commonplace objects.